Abstract
Introduction PAX5, a master regulator for B cell development, is observed with genetic alterations in over 30% of B cell Acute Lymphoblastic Leukemia (B-ALL). Point mutations, which highly enriched in the DNA binding domain, are the second most common alterations. Recently, PAX5 mutations have been recognized as founder events in multiple B-ALL subtypes, which account for over 10% of B-ALL. However, the molecular features and underlying mechanisms of the mutations in B-ALL are still unknown, which leaves the patients with generic therapy and poor outcome.
Methods To study the function of PAX5 mutations in B-ALL, we performed flow cytometry, RNA-seq, and CUT&Tag experiments in cell lines, transduced Pax5-/- B cells, and patient samples. In vitro differentiation and in vivo transplantation were conducted to assess whether these PAX5 mutants can affect B cell differentiation and transformation. Knock-in mouse strains were generated using CRISPR/Cas9 genome editing. In vivo bone marrow aspiration, single-cell (sc) RNA-seq, scBCR-seq, and sc-targeted genotyping were used to study the stepwise mutagenesis and B cell transformation process.
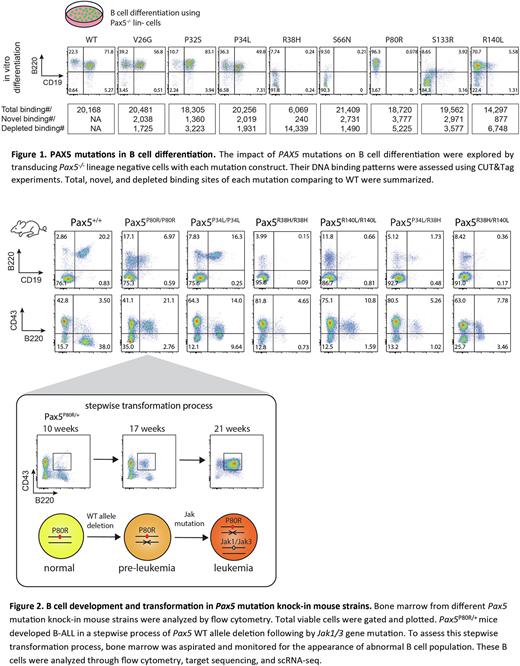
Results PAX5 P80R defines a B-ALL subtype with distinct gene expression profile (GEP). With the Pro80 replaced by Arg at the minor groove of DNA, PAX5 has been changed to acquire a greater number of novel bindings comparing to other mutations (Fig 1), yielded with 23% of differentially expressed genes in the P80R subtype have differential PAX5 binding. P80R binds to an intragenic promoter of MEGF10, a marker gene uniquely activated in the P80R subtype. Knock-down and CUT&Tag experiments showed that MEGF10 activation is likely induced by P80R binding and essential for maintaining malignant cell proliferation. In addition, loss of P80R binding in the WAPL promoter disinhibits WAPL gene, leading to the preferential usage of proximal V genes in the IgH locus. In vitro cultured P80R mutated B cells gain immortality while WT B cells enter senescence within 3 weeks. Finally, P80R is the only mutation tested that can support the growth of BAF3 cells independent of IL3.
B cell development in Pax5P80R/+ young miceis indistinguishable from WT, but it is blocked at pro-B stage in Pax5P80R/P80R mice. These mice developed B-ALL with full penetrance. All Pax5P80R/+ leukemic mice (n=53) have Pax5 WT allele deletion. Restoring WT PAX5 in P80R leukemia cells decreases their proliferation rate. However, P80R transduced Pax5-/- cells cannot induce B-ALL in sub-lethally irradiated mice, suggesting that P80R alone is prerequisite but not sufficient. Highly recurrent secondary Jak1/3 mutations were observed in P80R mouse leukemia. To test whether P80R combined with Jak mutation is sufficient, Pax5-/- cells were co-transduced with P80R and Jak3 R653H. Interestingly, this combination induced B-ALL in all recipient mice (n=6). Single-cell analysis of the GEP clusters, V(D)J recombination, and P80R genotypes of Pax5P80R/+ bone marrow revealed the stepwise WT allele deletion and Jak1/3 mutagenesis (Fig 2). Two major leukemic clones carrying different BCR light-chains were observed in one mouse, which were probably driven by different Jak mutations.
CUT&Tag of other recurrent PAX5 mutations revealed that R38H losses the majority while R140L losses one third of the WT binding sites (Fig 1). When co-transduced as commonly seen in patient B-ALL, R140L out-competes R38H for DNA binding. Consistently, R38H totally blocks B cell development while P80R and R140L arrest B cells at the pro-B stage (Fig 1). V26G, P32S and P34L do not significantly affect B cell development, consistent with their similar binding patterns to the WT. We generated knocked in mouse models for P34L, R38H, and R140L. In PAX5R38H/R38H mice, B cells are barely found. In PAX5R140L/R140L mice, B cells are decreased and blocked at the pro-B stage. PAX5P34L/P34L doesn't significantly affect B cell development. To recapitulate the co-occurrence of PAX5 mutations in patients, R38H/P34L and R38H/R140L mice were generated. They both block B cell development and decrease B cell population (Fig 2). Whether these mutations can act as driver events are still under investigation.
Conclusions Recurrent mutations in the DNA binding domain of PAX5 can lead to abnormal binding activities and deregulated gene expression patterns, which block normal B-cell differentiation and lead to overt B-ALL with the acquisition of additional genetic lesions.
Disclosures
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.